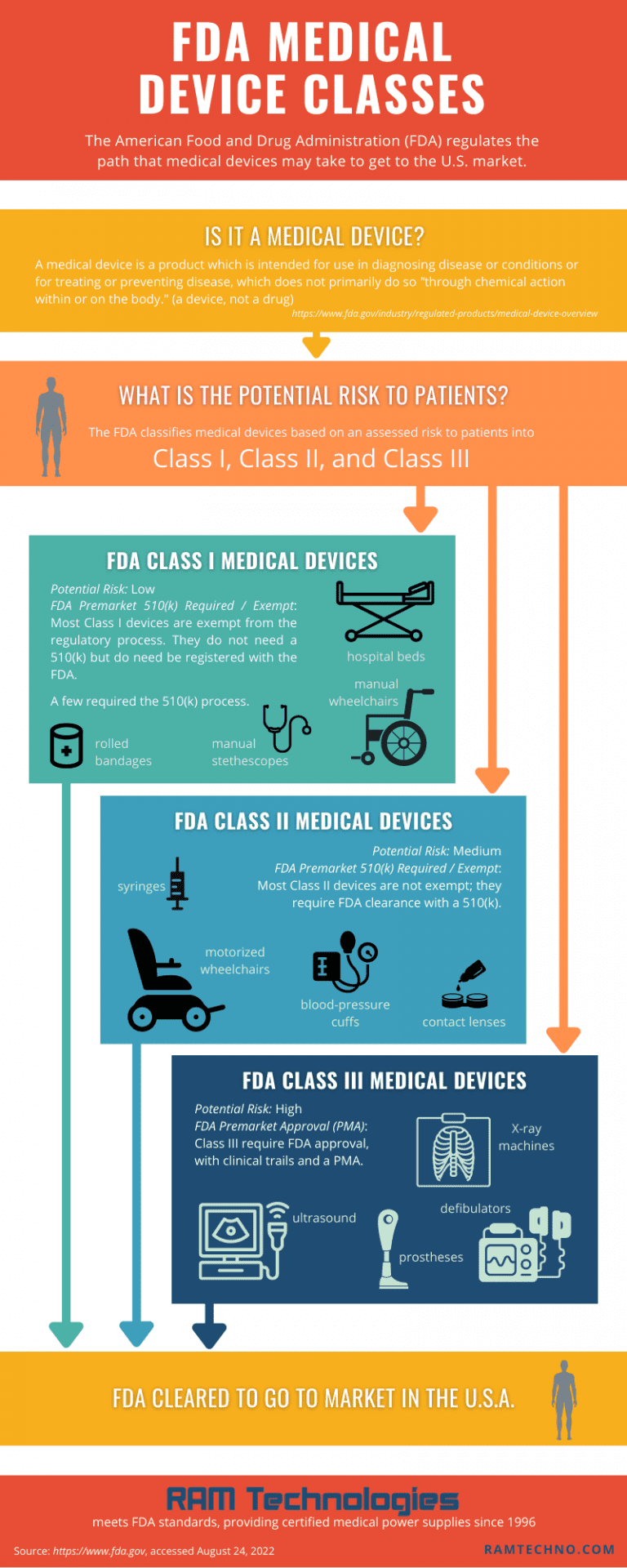
What Are The Fda Medical Device Classifications . Each class level carries a different set of regulations and requirements; The ranking is based primarily on the level of risk posed to the end user. classification of medical devices. Understanding the different medical device classes will be invaluable to bringing new products to market. Class i, class ii, and class iii. the fda categorized medical devices into three classes: Fda, the european commission, and health canada. fda has classified and described over 1,700 distinct types of devices and organized them in the cfr into 16 medical specialty. Medical device classes are a tiered categorization. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. The higher the class, the higher the risk. the openfda device classification api contains medical device names, their associated product codes, their medical. fda divides medical devices into three groups, class i, class ii, and class iii.
from ramtechno.com
The ranking is based primarily on the level of risk posed to the end user. the fda categorized medical devices into three classes: the openfda device classification api contains medical device names, their associated product codes, their medical. Understanding the different medical device classes will be invaluable to bringing new products to market. classification of medical devices. Medical device classes are a tiered categorization. Fda, the european commission, and health canada. fda divides medical devices into three groups, class i, class ii, and class iii. Each class level carries a different set of regulations and requirements; Class i, class ii, and class iii.
What Are the Three FDA Classes for Medical Devices? RAM Technologies
What Are The Fda Medical Device Classifications Each class level carries a different set of regulations and requirements; Fda, the european commission, and health canada. the fda categorized medical devices into three classes: Understanding the different medical device classes will be invaluable to bringing new products to market. The higher the class, the higher the risk. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. fda divides medical devices into three groups, class i, class ii, and class iii. Medical device classes are a tiered categorization. Class i, class ii, and class iii. the openfda device classification api contains medical device names, their associated product codes, their medical. classification of medical devices. Each class level carries a different set of regulations and requirements; The ranking is based primarily on the level of risk posed to the end user. fda has classified and described over 1,700 distinct types of devices and organized them in the cfr into 16 medical specialty.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification What Are The Fda Medical Device Classifications Each class level carries a different set of regulations and requirements; the openfda device classification api contains medical device names, their associated product codes, their medical. Fda, the european commission, and health canada. Understanding the different medical device classes will be invaluable to bringing new products to market. classification of medical devices. the fda categorized medical devices. What Are The Fda Medical Device Classifications.
From www.researchgate.net
Medical device classifications Download Scientific Diagram What Are The Fda Medical Device Classifications fda has classified and described over 1,700 distinct types of devices and organized them in the cfr into 16 medical specialty. the fda categorized medical devices into three classes: Fda, the european commission, and health canada. Each class level carries a different set of regulations and requirements; the openfda device classification api contains medical device names, their. What Are The Fda Medical Device Classifications.
From ramtechno.com
What Are the Three FDA Classes for Medical Devices? RAM Technologies What Are The Fda Medical Device Classifications fda has classified and described over 1,700 distinct types of devices and organized them in the cfr into 16 medical specialty. Understanding the different medical device classes will be invaluable to bringing new products to market. Each class level carries a different set of regulations and requirements; The higher the class, the higher the risk. the openfda device. What Are The Fda Medical Device Classifications.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS What Are The Fda Medical Device Classifications the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. Medical device classes are a tiered categorization. fda divides medical devices into three groups, class i, class ii, and class iii. Fda, the european commission, and health canada. Each class level carries a different set of regulations and requirements; The higher. What Are The Fda Medical Device Classifications.
From www.greenlight.guru
Understanding the FDA Medical Device Classification System What Are The Fda Medical Device Classifications the fda categorized medical devices into three classes: The higher the class, the higher the risk. classification of medical devices. Medical device classes are a tiered categorization. Each class level carries a different set of regulations and requirements; The ranking is based primarily on the level of risk posed to the end user. Class i, class ii, and. What Are The Fda Medical Device Classifications.
From healthtrustpg.com
FDA Approval Update HealthTrust Performance Improvement For Healthcare What Are The Fda Medical Device Classifications fda divides medical devices into three groups, class i, class ii, and class iii. Understanding the different medical device classes will be invaluable to bringing new products to market. Fda, the european commission, and health canada. the fda categorized medical devices into three classes: The ranking is based primarily on the level of risk posed to the end. What Are The Fda Medical Device Classifications.
From odoman.com
The 3 FDA Medical Device Classes [Differences and Examples Explained What Are The Fda Medical Device Classifications classification of medical devices. Each class level carries a different set of regulations and requirements; The higher the class, the higher the risk. The ranking is based primarily on the level of risk posed to the end user. fda divides medical devices into three groups, class i, class ii, and class iii. Class i, class ii, and class. What Are The Fda Medical Device Classifications.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero What Are The Fda Medical Device Classifications fda divides medical devices into three groups, class i, class ii, and class iii. Class i, class ii, and class iii. Fda, the european commission, and health canada. the openfda device classification api contains medical device names, their associated product codes, their medical. The higher the class, the higher the risk. the fda categorized medical devices into. What Are The Fda Medical Device Classifications.
From www.reghelps.com
FDA Medical Device Classification & Regulatory Controls What Are The Fda Medical Device Classifications fda has classified and described over 1,700 distinct types of devices and organized them in the cfr into 16 medical specialty. classification of medical devices. Class i, class ii, and class iii. Each class level carries a different set of regulations and requirements; the fda categorized medical devices into three classes: the food and drug administration. What Are The Fda Medical Device Classifications.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations What Are The Fda Medical Device Classifications the openfda device classification api contains medical device names, their associated product codes, their medical. the fda categorized medical devices into three classes: Each class level carries a different set of regulations and requirements; The ranking is based primarily on the level of risk posed to the end user. Class i, class ii, and class iii. fda. What Are The Fda Medical Device Classifications.
From www.pinterest.com
Medical Device Classification and FDA Approval Process Medical What Are The Fda Medical Device Classifications Fda, the european commission, and health canada. the fda categorized medical devices into three classes: The higher the class, the higher the risk. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. Each class level carries a different set of regulations and requirements; fda has classified and described over. What Are The Fda Medical Device Classifications.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co What Are The Fda Medical Device Classifications the fda categorized medical devices into three classes: Understanding the different medical device classes will be invaluable to bringing new products to market. Class i, class ii, and class iii. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. The ranking is based primarily on the level of risk posed. What Are The Fda Medical Device Classifications.
From synectic.net
Medical Device FDA Regulations Infographic Synectic What Are The Fda Medical Device Classifications the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. Class i, class ii, and class iii. The higher the class, the higher the risk. fda has classified and described over 1,700 distinct types of devices and organized them in the cfr into 16 medical specialty. classification of medical devices.. What Are The Fda Medical Device Classifications.
From www.reghelps.com
FDA Medical Device Classification & Regulatory Controls What Are The Fda Medical Device Classifications fda divides medical devices into three groups, class i, class ii, and class iii. Understanding the different medical device classes will be invaluable to bringing new products to market. Medical device classes are a tiered categorization. Each class level carries a different set of regulations and requirements; The higher the class, the higher the risk. the openfda device. What Are The Fda Medical Device Classifications.
From www.jamasoftware.com
FDA Medical Device Class and Classifications Jama Software What Are The Fda Medical Device Classifications Medical device classes are a tiered categorization. classification of medical devices. The ranking is based primarily on the level of risk posed to the end user. Fda, the european commission, and health canada. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. fda has classified and described over 1,700. What Are The Fda Medical Device Classifications.
From www.jamasoftware.com
FDA Medical Device Class and Classifications Jama Software What Are The Fda Medical Device Classifications the openfda device classification api contains medical device names, their associated product codes, their medical. Fda, the european commission, and health canada. Each class level carries a different set of regulations and requirements; Class i, class ii, and class iii. Medical device classes are a tiered categorization. Understanding the different medical device classes will be invaluable to bringing new. What Are The Fda Medical Device Classifications.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co What Are The Fda Medical Device Classifications classification of medical devices. Each class level carries a different set of regulations and requirements; The ranking is based primarily on the level of risk posed to the end user. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. fda divides medical devices into three groups, class i, class. What Are The Fda Medical Device Classifications.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment What Are The Fda Medical Device Classifications Fda, the european commission, and health canada. Understanding the different medical device classes will be invaluable to bringing new products to market. classification of medical devices. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. fda has classified and described over 1,700 distinct types of devices and organized them. What Are The Fda Medical Device Classifications.